MLD is a rare, genetic, neurometabolic demyelinating lysosomal storage disorder caused by a deficiency of the arylsulfatase A (ARSA) enzyme due to mutations in the ARSA gene.1,2
MLD is inherited in an autosomal-recessive pattern – the overall carrier frequency being between 1:100 and 1:130.3


The ARSA gene encodes ARSA, a lysosomal enzyme necessary for the metabolism of sulfatides, a major component of the myelin membrane. Patients with MLD inherit two mutant alleles of the ARSA gene and have inadequate ARSA enzyme activity.
Damaging levels of sulfatides accumulate in lysosomes, leading to progressive demyelination that results in missed developmental milestones, regression, and ultimately leading to severe neurological symptoms.2 Patients experience severe motor and cognitive impairment, especially in the most common, early onset forms of the disease (late infantile and early juvenile forms).2


Symptoms, age on onset and disease course vary, but patients progress to dysphagia, severe neurological disability and death.1,2
MLD has a considerable impact on the social, emotional and professional lives of patients and their families, including an average of 17 hours per day spent by families caring for their child with MLD.8
The global incidence of MLD is ~1.6 (range 0.6–2.5)/100,000 live births (for all forms of MLD).9
Diagnosing MLD is a challenge for several reasons:10
Early identification is critical. The progressive, irreversible nature of MLD demands an understanding of its clinical course and requires immediate, decisive action to prevent patient regression and improve overall outcomes.7
Early symptoms vary depending on whether the condition is late-infantile (LI) or juvenile (JU) MLD.




For HCPs with a symptomatic patient, the following process should be followed:12

Qualified treatment centres (QTCs) have the required infrastructure and experience in haematopoietic stem cell transplantation and the management of leukodystrophies to ensure the consistency and quality of treatment. For more details, click here to see treatment process.
The earlier patients can be identified, the better the outcomes. This can be achieved by family screening and, ideally, newborn screening.12 Although newborn screening is not yet routinely implemented for MLD, pilot newborn screening studies are under way.

To allow a timely diagnosis and potential treatment, it is strongly recommended to initiate parallel family testing upon a strong suspicion of an MLD index case.12

Before the approval of Libmeldy, there were limited treatment options for patients with MLD.13-16
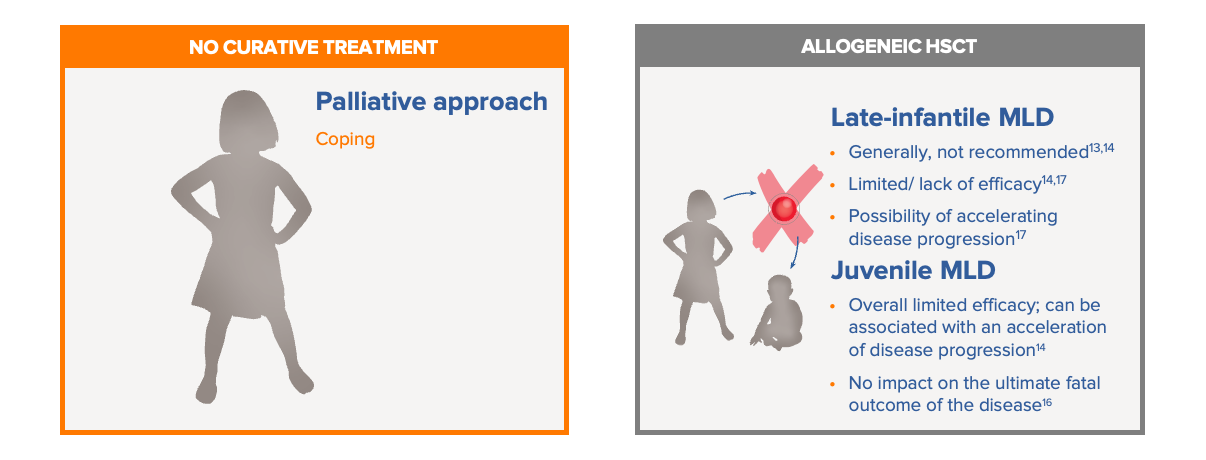
Standard of care in MLD previously was limited to best supportive care, which involves the palliative treatment of symptoms. The use of allogeneic HSCT is only used in a minority of juvenile MLD patients. For many patients with MLD, particularly those with the late infantile, pre-symptomatic and early symptomatic form of the disease, allogenic HSCT is not recommended due to limited/no efficacy and inherent risks (including the possibility that pre-transplant procedures may even facilitate disease progression).17 Limitations of HSCT may include risk of graft-vs-host disease, limited levels of ARSA produced by the bone marrow HCST-derived microglia, limited donor match availability, and procedure-associated complications. Best supportive care and allogeneic HSCT are summarised below:13-16
Libmeldy is the first autologous haematopoietic stem cell (HST) gene therapy product for early-onset MLD, as well as the first approved treatment.18

References
