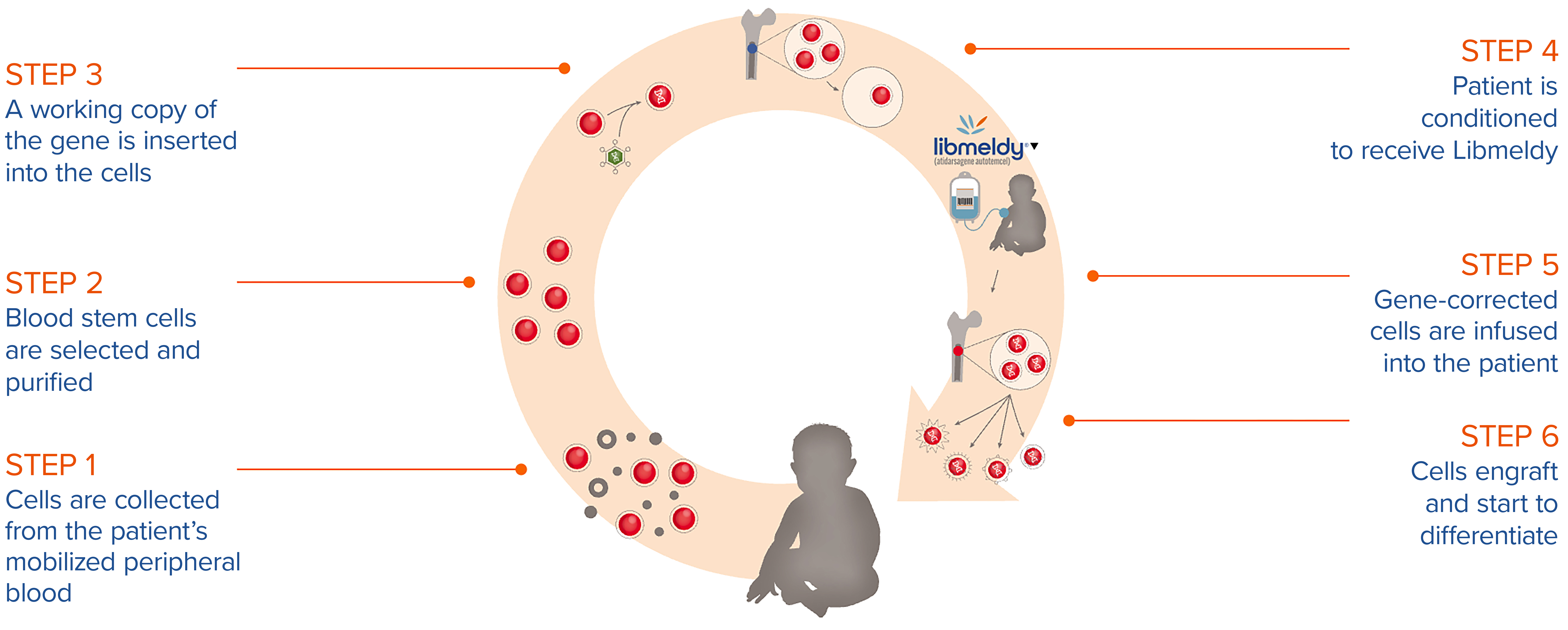
The process of manufacturing Libmeldy involves blood stem cells being selected from the patient, a working copy of the ARSA gene inserted and the gene-corrected cells infused back into the patient.


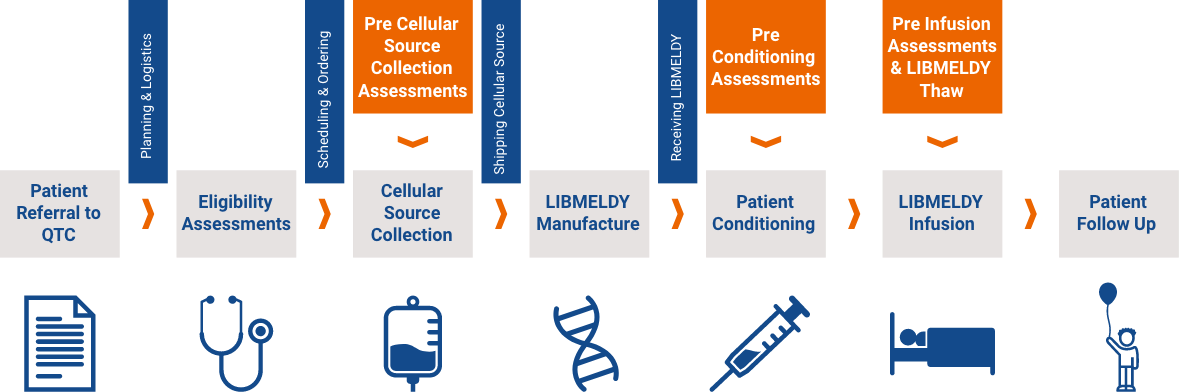



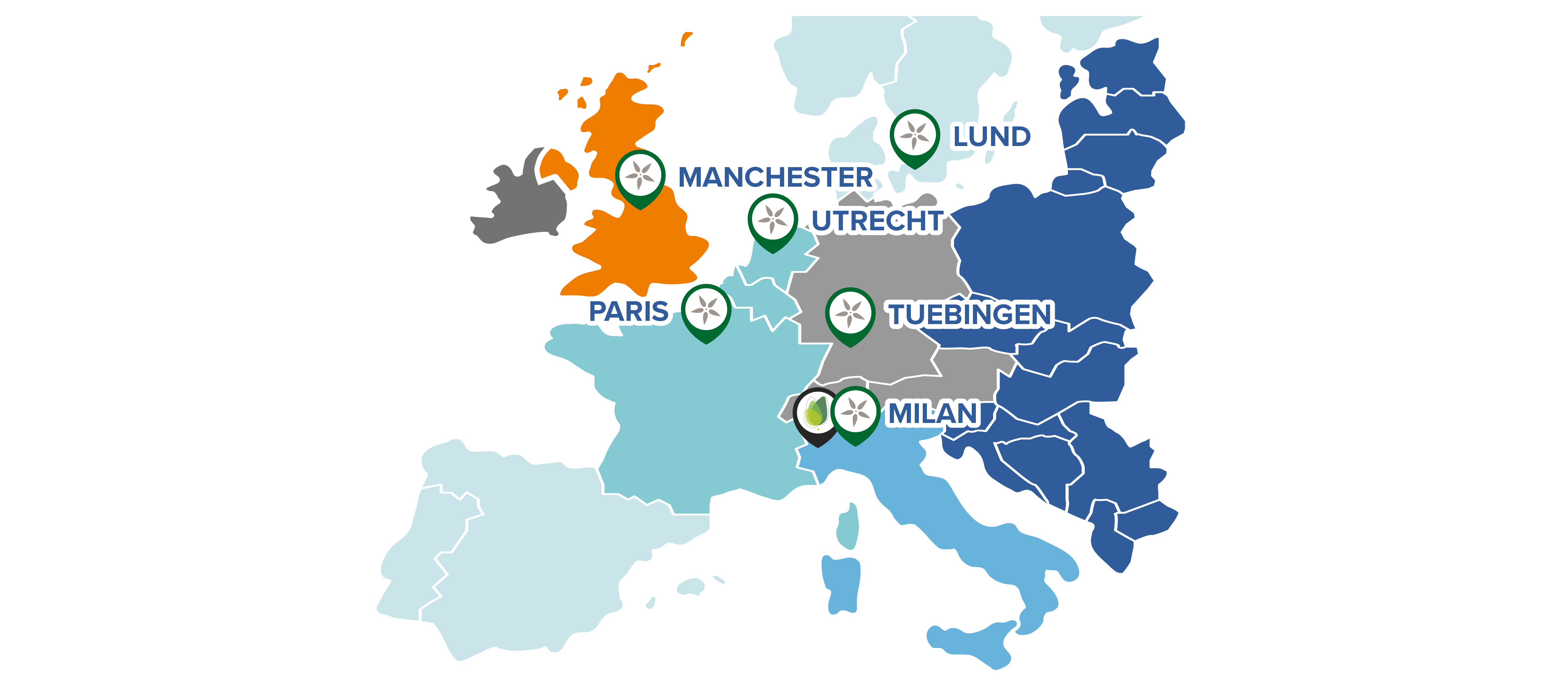


To ensure consistency and quality of treatment, Orchard Therapeutics has qualified six QTCs in Europe to administer Libmeldy. All are experienced in haematopoietic stem cell transplantation and the management of leukodystrophies.
All QTCs have the required infrastructure, procedures and accreditations in place for:

If you have identified an MLD patient in your clinic, please contact one of the Qualified Treatment Centres (QTCs) or Orchard Therapeutics. You can either contact your local Orchard Therapeutics representative directly or send an email to: info@orchard-tx.com